Mobile robots can dance around a stage, perform graceful acrobatics and even lift heavy objects. But if you watched them strut their stuff for an hour or two, you would see the robots grind to a halt. Like humans, mobile robots eventually exhaust the energy that they carry, and need a recharge.
This problem is specific to mobile robots. Robots anchored to a factory floor can do heavy work all day and all night because they can draw inexhaustible energy from the electric grid. Mobility gives robots more flexibility, but at the cost of needing to recharge their energy sources — in most cases, some form of battery.Part of Nature Outlook: Robotics and artificial intelligence
The compact nature of smartphones can fool us into thinking batteries are featherweight objects. That illusion arises because modern electronics need only a trickle of energy to send signals or process data. But transporting robots or people around, or lifting a heavy load, takes much more energy. If you pick up a cordless tool, you will feel that it outweighs a corded one. An electric car that can travel for five hours (around 500 kilometres, the distance from Paris to Amsterdam) at motorway speed between recharges needs batteries that account for one-third or more of the total vehicle weight.
Mobile robots on legs, however, can’t tolerate such massive batteries. Boston Dynamics, a robotics company in Waltham, Massachusetts, sells a four-legged dog-size robot called Spot that weighs about 32 kg — one-eighth of which is batteries. But, the company states it has a typical run-time of only 90 minutes. Humanoid robots that have been developed to walk with heavy loads have the same limitations. Atlas, the company’s 1.5 metre, 89-kilogram humanoid demonstrator with two arms and two legs, can do gymnastics and lift heavy objects. But the company does not say how long it can run before it needs a recharge. For mobile robots to be more capable workers, their batteries will need greater energy density — that is, they will need to pack more watt-hours of energy into fewer kilograms of mass. “Energy density is still quite far from the power we need for robotics,” says Ravinder Dahiya, an electrical engineer specializing in robotics at Northeastern University in Boston, Massachusetts.
How serious the energy-density problem is depends on the robot’s size and structure, its function and how much energy it needs. Robots that walk can navigate stairs, the interiors of buildings and rough terrain better than wheels — but they can’t carry as big of a battery pack. Sustained flight requires even more energy, making battery weight a serious limit for anything much bigger than insect size.
The limits of lithium
Batteries have come a long way since the Italian physicist Alessandro Volta invented the earliest version of this technology in 1800. Today, the state-of-the-art power source is the lithium-ion battery, invented in the 1970s by chemist Stanley Whittingham, and now widely used in phones, laptops, tools and electric vehicles. The technology earned Whittingham, now at the State University of New York at Binghamton, a share of the 2019 Nobel Prize in Chemistry.
Batteries don’t generate energy; they store energy produced by chemical reactions that yield positive ions and electrons. The ions accumulate at one end of the battery, called the cathode, and the electrons at the other end, called the anode. The ions and electrons sit on these two ends until they are connected by a conductor, which completes the circuit and allows the electrons to flow as a current from the anode to deliver electrical power to an externally connected load — such as an electric motor — and then to the cathode. When the chemicals are used up, the battery must either be replaced or recharged by passing a current through it in the opposite direction, to reverse the reaction.

A major advantage of batteries is that they directly deliver energy in the form of electricity, whereas fossil fuels have to be burned to generate heat that drives an electrical generator. This avoids carbon emissions on the spot, although total emissions depend on how the original energy was produced. However, robots must carry the energy they use, and batteries weigh more and occupy more space than fossil fuels. An electric car, for example, needs a battery pack much larger and heavier than a fuel tank.
When lithium-ion batteries reached the market in 1991, they provided 80 watt-hours of electrical energy per kilogram of battery weight1. That meant it took a one kilogram battery to power a (then standard) 60-watt incandescent bulb for one hour and 20 minutes, making it the best battery available. Now, typical commercial lithium-ion batteries carry three times more energy per kilogram. But even such energy-packed batteries are too hefty for a walking robot to lug around.
Chemistry quest
Lithium-ion batteries are running out of steam. The chemistry has “less and less room for improvement”, says Richard Schmuch, a chemist at the Fraunhofer Research Institution for Battery Cell Production in Münster, Germany. Lithium itself is rare and expensive. The same is true for cobalt, another crucial element which can make up to 20% of the weight of the cathode in lithium-ion batteries for electric vehicles. Extracting both elements requires large amounts of energy and water. Moreover, the mining of cobalt has been linked to the exploitation of workers.
Another concern is optimizing batteries to meet the needs of robotics. “The lithium-ion battery is quite versatile,” says Schmuch. “You can adjust it for different types of operating condition,” from smartphones to cars to robots. Yet it can’t do everything cost-effectively and well. He expects new types of battery will be needed to serve the emerging demands of robotics as well as other applications.
After more than 30 years of development, lithium-ion batteries are considered to be a mature technology. Still, efforts continue to improve these complex electrochemical systems. They are assembled in units called cells, which are packaged together to provide a desired electrical output. Each cell contains — in addition to the anode and cathode — an electrolyte through which ions can move, a separator to prevent short circuits and electrical terminals that connect to other cells in the packaged battery. Extensive research has gone into the composition of each part to achieve high energy density, charging and discharging rates, reliability and longevity. Among the important successes of this technology are batteries that can be recharged as many as 6,000 times.
One attempt to enhance the performance of lithium entails making the anode and cathode from nanostructured sulfur-graphite composites rather than from standard graphite. Such lithium–sulfur batteries offer the potential of lower costs and higher energy density. These batteries have yet to be commercialized successfully, however; their use might be limited to specialized applications, such as aviation, for which minimizing battery weight is crucial to get off the ground. Battery features could be tailored by adjusting design details, such as the type of nanostructure used and how the ions and electrons flow through the battery. But what many developers want is new battery chemistries designed to meet a variety of needs (see ‘Packing in the power’).
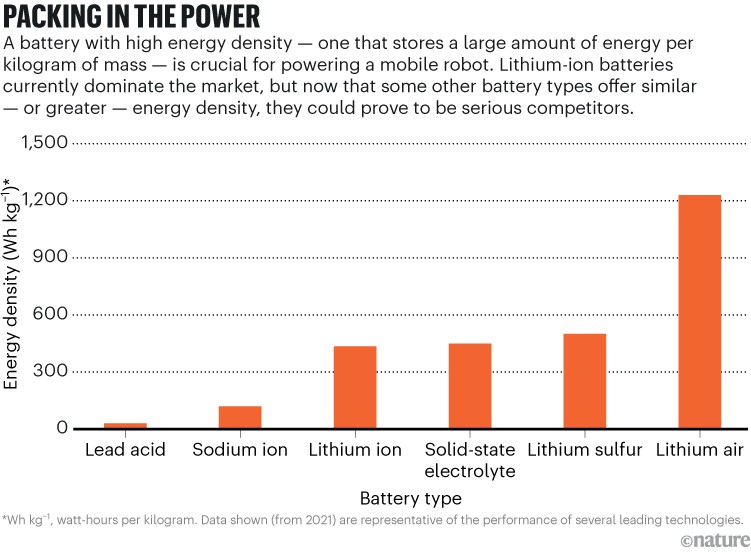
That might entail stepping back from lithium’s biggest attraction — it is the lightest metal among the elements, with an atomic weight of seven. Yet, although lithium is absolutely essential to the battery’s energy storage and release, other materials make up more of the battery’s mass. Including the packaging, only about 1% of the weight of a lithium-ion battery is lithium (most of that in the cathode). The cathode also contains more of four other metals: cobalt, nickel, aluminium and manganese. Several problems with lithium and cobalt have led to serious interest in sodium-ion batteries.
Like lithium, sodium is an alkali metal, and the chemistry of the two is so similar that researchers have pursued sodium-ion batteries as a way around the problems with lithium. One important advantage of the sodium-ion design is the ready availability of sodium in seawater and salt deposits — avoiding the supply-chain problems arising from the cost and scarcity of lithium. Sodium-ion batteries are a bit heavier per kilowatt-hour of energy — sodium’s atomic weight is 23, more than triple that of lithium. Still, lower material costs are expected to make sodium-ion batteries significantly cheaper. An even bigger benefit of switching to sodium would come from reducing or eliminating the need for cobalt in the cathode, which has been demonstrated in several samples.
“Sodium ions are definitely gaining traction,” says Schmuch, citing development of them in Germany, where Fraunhofer is working with industry, and in China, where Contemporary Amperex Technology (CATL) in Ningde — the world’s leading manufacturer of lithium-ion batteries for electric vehicles — rolled out the first generation of its sodium-ion battery in 2021. This April, Chery Automobile in Wuhu, China, announced plans to install CATL sodium-ion batteries in its cars. Also in April, CATL said it had developed a new electric vehicle battery with an energy density of 500 watt-hours per kilogram. This battery employs a different technology, which CATL have not identified.
Solid-state solutions
Another way to change battery chemistry is to change the state of the electrolytes, replacing the conductive liquids used in lithium-ion batteries with conductive solids. Advocates think such solid-state batteries offer the best prospects for preventing the potentially deadly fires seen with lithium batteries, as well as for improving energy density and reducing costs.
The fire hazard comes from filamentary deposits of metallic lithium called dendrites that grow in electrolytes in the batteries. Lithium atoms present in the solvent crystallize to form metal filaments that spread like plant roots. The metallic lithium is conductive, and as the dendrites spread they can short circuit the battery and ignite fires. Sodium is much less prone to dendrite formation, and developers think that this quality makes sodium-ion batteries significantly safer than lithium-ion batteries. They would also be lower cost and in the long term potentially offer higher energy density.
A wide range of solid-state batteries are in development. Lithium is still a popular material because of its light weight, high energy density and rechargeability. But some researchers are exploring other metals with the hope of avoiding the known problems of lithium.

Switching one type of lithium battery in particular from liquid to solid-state electrolytes has led to a big advance in efficiency. This beneficiary is the lithium-air battery, which produces power from the oxidation of lithium atoms by oxygen from the air. As with sodium-ion batteries, energy density was not the main goal for many people working on solid-state batteries. “The reason for developing a solid-state electrolyte was to make the lithium-air battery more safe and to make recharging cycles more stable,” says Mohammad Asadi, a chemical engineer at the Illinois Institute of Technology in Chicago.
However, when Asadi and his colleagues from Argonne National Laboratory in Lemont, Illinois, built an experimental solid-state lithium-air battery2, they were surprised to discover that the technology brought significant benefits in energy density as well. The device transferred four electrons per reaction rather than one or two electrons per reaction that lithium-air batteries normally produce. As a result, Asadi says, the solid electrolyte “helps us store three to four times more energy per unit weight” than is possible with conventional lithium-ion batteries.
In fact, their new solid electrolyte changed the chemistry between oxygen and lithium. In standard lithium-air batteries, oxygen molecules from the air react with lithium atoms to produce one of two compounds. The reaction between one lithium atom and one oxygen molecule produces lithium superoxide (LiO2), which yields one electron. The reaction between two lithium atoms and one oxygen molecule produces lithium peroxide (Li2O2), which yields two electrons.
Asadi’s team made its solid electrolyte by combining nanoparticles containing lithium, germanium, phosphorous and sulfur (Li10GeP2S12) with a polymer. In this structure, two lithium atoms can combine with a single oxygen atom to yield lithium oxide (Li2O) and four electrons. That’s hard to do because it requires splitting an oxygen molecule (O2) to produce a single oxygen atom. The test cell, only the size of a coin, was a proof of concept. According to Asadi, this prototype shows that it will be possible to attain a specific energy of one kilowatt-hour per kilogram — higher than is possible with today’s lithium-ion technology.
Power-studded structures
Increasing the energy density of batteries makes it possible for these power packs to weigh less, which in turn would allow mobile robots of a given size to do more work. Battery design for such robots involves much more than chemistry. And one way to realize this would be to have smaller batteries serve as structural elements of a robot — not just storing energy but also becoming parts of its torso and legs to help it walk and balance. The idea comes from biology. Our bones are not just structural support — they also contain bone marrow that produces blood cells. “Multifunctionality is critical” when you’re building robots that move around, says Nicholas Kotov, a chemical engineer at the University of Michigan in Ann Arbor.
“Robots are biomimetic, and the smaller the robot, the more biological concepts would need to be there,” Kotov says. He and other roboticists call two-legged robots humanoid because they walk upright and have similar body mechanics to people. “We want to keep robots light and consuming as small as an amount of energy as possible,” Kotov says. “If a battery just sits there and does nothing else [but provide power], it is not enough.”
Kotov’s group is particularly interested in drones, in which, he says “every gram counts, and if the battery can serve multiple functions, we can have more functional space”. His team is now working on structural batteries for military drones, although not much of that work has been disclosed yet. Military laboratories have also worked on humanoid robots for missions such as working inside radiation zones and checking for insurgents hiding inside buildings in combat zones.
Some materials used for battery energy storage are particularly well-suited for also being structural elements. For example, Kotov says, “zinc is a very good case for structural batteries”. It is inexpensive, stores energy well and the metal is stable in air. His lab demonstrated a biomorphic zinc battery that could store 72 times more energy than a lithium battery of the same volume3. However, trade-offs are
inevitable. Zinc batteries have limited rechargeability, so they would be best kept stashed away for infrequent use.Another promising multifunctional material is aluminium, which, last year, showed rapid rechargeability over hundreds of cycles at temperatures up to just above the boiling point of water — and without forming aluminium dendrites4. The researchers project a cost of less than one-sixth of that of comparable lithium-ion batteries with a similar energy capacity.
Kotov is also developing aramid fibres to provide structural strength for battery casings and internal battery structures3. These fibres have a fortunate combination of features including strength, flexibility and hardness that makes them useful for protective shielding. One particularly helpful attribute is their ability to block dendrites from growing between the electrodes. Moreover, aramid offers an environmental advantage — it can be made from recycled Kevlar, a strong, lightweight material, and when the batteries are worn out, the fibres can be recycled for further uses.
Energy beyond batteries
By 2030, Dahiya expects the development of energy sources for mobile robotics to broaden well beyond batteries. Some of these concepts have their roots in biology.
One example is equipping robots that operate at remote sites with energy harvesters, to collect energy from their local environment to top up their stored energy5. Robots can collect energy in the form of radio waves or sunlight, or from a thermal gradient. However, energy harvesting is not as efficient as heat pumps or wireless chargers, but it can operate in any suitable environment without special charging equipment. And there have been demonstrations of tiny bacterial-driven microbial fuel cells, or ‘biobatteries’, that could harvest material from the local biota to provide supplemental power6.
Another concept borrowed from biology is distributing energy in various ways through the robot’s body rather than concentrating it in a single battery backpack as used on some experimental humanoid robots5. Humans have three types of energy storage: triglycerides in fat cells, glycogen clusters around muscles and ATP that’s produced by mitochondria. Those systems evolved to serve different energy needs. “Humans and animals require fast energy and slow energy,” says Kotov. They need the fast energy to sprint, as well as slow energy to walk for many kilometres.
Robots and drones likewise have different needs at different times. A humanoid robot needs fast energy to lift a heavy load or run up stairs, and slower energy to patrol a field or a car park. Batteries are fine for a steady walk or jog, but not for a sprint. This gap has led to an interest in equipping robots with a different type of device — a supercapacitor — that delivers electrical energy much faster. Instead of using chemistry to store energy, a supercapacitor stores an electrical charge that it collects over a period of time from an electrical circuit. When a burst of energy is needed, the system discharges the stored electrons extremely quickly. Supercapacitors are used in regenerative braking systems in vehicles and can withstand many more charge–discharge cycles than can batteries7. In the future, they could give mobile robots a quick start — or a quick stop.
The lithium-ion generation of batteries put smartphones in our pockets and electric cars on our roads. Researchers are now in the early stages of developing a generation of portable energy sources that will be lighter, more efficient and potentially cheaper.
Yet costs might still be troublesome, cautions materials chemist Donald Sadoway at the Massachusetts Institute of Technology in Cambridge. Sadoway, who focuses on energy-storage technologies, sees little interest in “new battery chemistries whose price-to-performance ratio is less favourable than that of today’s lithium-ion”. It is unclear, he says, whether “the commercial opportunity is large enough to attract the investment needed for the requisite research and development to invent the new technology and to bring it to market”.
The prospects look bright to material scientist Shirley Meng, who is chief scientist at the Argonne Collaborative Center for Energy Storage Science at Argonne National Laboratory. She says using the oxygen from the air as a cathode is “the ultimate dream of battery scientists” as it offers high energy with light weight. “Good progress has been made” on the lithium-air battery, on which Argonne collaborated, but she says “we still face a lot of challenges in understanding and overcoming the limiting factors in enabling air cathodes”.
Meng predicts that the sodium battery, free of elements such as lithium, nickel and cobalt, “will find its niche to shine” because of its high ratio of performance to cost. Solid-state batteries, “offer the possibility of achieving the highest volumetric energy density in robotic applications [in which] space is limited”, she says. They also offer unique flexibility in packaging and can operate at extreme temperatures, which is important for some special-purpose robots. Meng is optimistic that developers “will offer a wide variety of battery solutions to different types of robot application”, with the potential “to unlock applications that were previously not possible”.
doi: https://doi.org/10.1038/d41586-023-02170-y
This article is part of Nature Outlook: Robotics and artificial intelligence, an editorially independent supplement produced with the financial support of third parties. About this content.
References
- Duffner, F., Kronemeyer, N., Tübke, J., Leker, J., Winter, M. & Schmuch, R. Nature Energy 6, 123–134 (2021).Article Google Scholar
- Kondori, A. et al. Science 379, 499–505 (2023).Article PubMed Google Scholar
- Wang, M. et al. Sci. Robot. 5, eaba1912 (2020).Article PubMed Google Scholar
- Pang, Q. et al. Nature 608, 704–711 (2022).Article PubMed Google Scholar
- Mukherjee, R., Ganguly, P. & Dahiya, R. Adv. Intell. Syst. 5, 2100036 (2023).Article Google Scholar
- Gao, Y., Mohammadifar, M. & Choi, S. Adv. Mater. Technol. 4, 1900079 (2019).Article Google Scholar
- Partridge, J. & Ibrahim Abouelamaimen, D. Energies 12, 2683 (2019).





