This article was authored by Anthony King and originally published to Nature
Cancer drugs usually take a scattergun approach. Chemotherapies inevitably hit healthy bystander cells while blasting tumours, sparking a slew of side effects. It is also a big ask for an anticancer drug to find and destroy an entire tumour — some are difficult to reach, or hard to penetrate once located.
A long-dreamed-of alternative is to inject a battalion of tiny robots into a person with cancer. These miniature machines could navigate directly to a tumour and smartly deploy a therapeutic payload right where it is needed. “It is very difficult for drugs to penetrate through biological barriers, such as the blood–brain barrier or mucus of the gut, but a microrobot can do that,” says Wei Gao, a medical engineer at the California Institute of Technology in Pasadena.
Part of Nature Outlook: Robotics and artificial intelligence
Among his inspirations is the 1966 film Fantastic Voyage, in which a miniaturized submarine goes on a mission to remove a blood clot in a scientist’s brain, piloted through the bloodstream by a similarly shrunken crew. Although most of the film remains firmly in the realm of science fiction, progress on miniature medical machines in the past ten years has seen experiments move into animals for the first time.
There are now numerous micrometre- and nanometre-scale robots that can propel themselves through biological media, such as the matrix between cells and the contents of the gastrointestinal tract. Some are moved and steered by outside forces, such as magnetic fields and ultrasound. Others are driven by onboard chemical engines, and some are even built on top of bacteria and human cells to take advantage of those cells’ inbuilt ability to get around. Whatever the source of propulsion, it is hoped that these tiny robots will be able to deliver therapies to places that a drug alone might not be able to reach, such as into the centre of solid tumours. However, even as those working on medical nano- and microrobots begin to collaborate more closely with clinicians, it is clear that the technology still has a long way to go on its fantastic journey towards the clinic.

Poetry in motion
One of the key challenges for a robot operating inside the human body is getting around. In Fantastic Voyage, the crew uses blood vessels to move through the body. However, it is here that reality must immediately diverge from fiction. “I love the movie,” says roboticist Bradley Nelson, gesturing to a copy of it in his office at the Swiss Federal Institute of Technology (ETH) Zurich in Switzerland. “But the physics are terrible.” Tiny robots would have severe difficulty swimming against the flow of blood, he says. Instead, they will initially be administered locally, then move towards their targets over short distances.
When it comes to design, size matters. “Propulsion through biological media becomes a lot easier as you get smaller, as below a micron bots slip between the network of macromolecules,” says Peer Fischer, a robotics researcher at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. Bots are therefore typically no more than 1–2 micrometres across. However, most do not fall below 300 nanometres. Beyond that size, it becomes more challenging to detect and track them in biological media, as well as more difficult to generate sufficient force to move them.
Scientists have several choices for how to get their bots moving. Some opt to provide power externally. For instance, in 2009, Fischer — who was working at Harvard University in Cambridge, Massachusetts, at the time, alongside fellow nanoroboticist Ambarish Ghosh — devised a glass propeller, just 1–2 micrometres in length, that could be rotated by a magnetic field1. This allowed the structure to move through water, and by adjusting the magnetic field, it could be steered with micrometre precision. In a 2018 study2, Fischer launched a swarm of micropropellers into a pig’s eye in vitro, and had them travel over centimetre distances through the gel-like vitreous humour into the retina — a rare demonstration of propulsion through real tissue. The swarm was able to slip through the network of biopolymers within the vitreous humour thanks in part to a silicone oil and fluorocarbon coating applied to each propeller. Inspired by the slippery surface that the carnivorous pitcher plant Nepenthes uses to catch insects, this minimized interactions between the micropropellers and biopolymers.
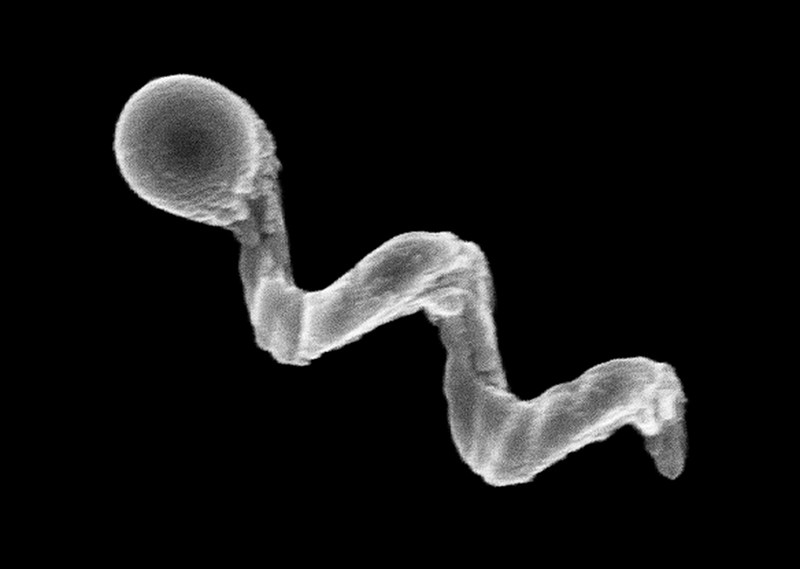
Another way to provide propulsion from outside the body is to use ultrasound. One group placed magnetic cores inside the membranes of red blood cells3, which also carried photoreactive compounds and oxygen. The cells’ distinctive biconcave shape and greater density than other blood components allowed them to be propelled using ultrasonic energy, with an external magnetic field acting on the metallic core to provide steering. Once the bots are in position, light can excite the photosensitive compound, which transfers energy to the oxygen and generates reactive oxygen species to damage cancer cells.
This hijacking of cells is proving to have therapeutic merits in other research projects. Some of the most promising strategies aimed at treating solid tumours involve human cells and other single-celled organisms jazzed up with synthetic parts. In Germany, a group led by Oliver Schmidt, a nanoscientist at Chemnitz University of Technology, has designed a biohybrid robot based on sperm cells4. These are some of the fastest motile cells, capable of hitting speeds of 5 millimetres per minute, Schmidt says. The hope is that these powerful swimmers can be harnessed to deliver drugs to tumours in the female reproductive tract, guided by magnetic fields. Already, it has been shown that they can be magnetically guided to a model tumour in a dish.
Credit: Leibniz IFW, Dresden
“We could load anticancer drugs efficiently into the head of the sperm, into the DNA,” says Schmidt. “Then the sperm can fuse with other cells when it pushes against them.” At the Chinese University of Hong Kong, meanwhile, nanoroboticist Li Zhang led the creation of microswimmers from Spirulina microalgae cloaked in the mineral magnetite. The team then tracked a swarm of them inside rodent stomachs using magnetic resonance imaging5. The biohybrids were shown to selectively target cancer cells. They also gradually degrade, reducing unwanted toxicity.
Another way to get micro- and nanobots moving is to fit them with a chemical engine: a catalyst drives a chemical reaction, creating a gradient on one side of the machine to generate propulsion. Samuel Sánchez, a chemist at the Institute for Bioengineering of Catalonia in Barcelona, Spain, is developing nanomotors driven by chemical reactions for use in treating bladder cancer. Some early devices relied on hydrogen peroxide as a fuel. Its breakdown, promoted by platinum, generated water and oxygen gas bubbles for propulsion. But hydrogen peroxide is toxic to cells even in minuscule amounts, so Sánchez has transitioned towards safer materials. His latest nanomotors are made up of honeycombed silica nanoparticles, tiny gold particles and the enzyme urease6. These 300–400-nm bots are driven forwards by the chemical breakdown of urea in the bladder into carbon dioxide and ammonia, and have been tested in the bladders of mice. “We can now move them and see them inside a living system,” says Sánchez.
Breaking through
A standard treatment for bladder cancer is surgery, followed by immunotherapy in the form of an infusion of a weakened strain of Mycobacterium bovis bacteria into the bladder, to prevent recurrence. The bacterium activates the person’s immune system, and is also the basis of the BCG vaccine for tuberculosis. “The clinicians tell us that this is one of the few things that has not changed over the past 60 years,” says Sánchez. There is a need to improve on BCG in oncology, according to his collaborator, urologic oncologist Antoni Vilaseca at the Hospital Clinic of Barcelona. Current treatments reduce recurrences and progression, “but we have not improved survival”, Vilaseca says. “Our patients are still dying.”
The nanobot approach that Sánchez is trying promises precision delivery. He plans to insert his bots into the bladder (or intravenously), to motor towards the cancer with their cargo of therapeutic agents to target cancer cells, using abundant urea as a fuel. He might use a magnetic field for guidance, if needed, but a more straightforward replacement of BCG with bots that do not require external control, perhaps using an antibody to bind a tumour marker, would please clinicians most. “If we can deliver our treatment to the tumour cells only, then we can reduce side effects and increase activity,” says Vilaseca.
Not all cancers can be reached by swimming through liquid, however. Natural physiological barriers can block efficient drug delivery. The gut wall, for example, allows absorption of nutrients into the bloodstream, and offers an avenue for getting therapies into bodies. “The gastrointestinal tract is the gateway to our body,” says Joseph Wang, a nanoengineer at the University of California, San Diego. However, a combination of cells, microbes and mucus stops many particles from accessing the rest of the body. To deliver some therapies, simply being in the intestine isn’t enough — they also need to be able to burrow through its defences to reach the bloodstream, and a nanomachine could help with this.
In 2015, Wang and his colleagues, including Gao, reported the first self-propelled robot in vivo, inside a mouse stomach7. Their zinc-based nanomotor dissolved in the harsh stomach acids, producing hydrogen bubbles that rocketed the robot forwards. In the lower gastrointestinal tract, they instead use magnesium. “Magnesium reacts with water to give a hydrogen bubble,” says Wang. In either case, the metal micromotors are encapsulated in a coating that dissolves at the right location, freeing the micromotor to propel the bot into the mucous wall.
Some bacteria have already worked out their own ways to sneak through the gut wall. Helicobacter pylori, which causes inflammation in the stomach, excretes urease enzymes to generate ammonia and liquefy the thick mucous that lines the stomach wall. Fischer envisages future micro- and nanorobots borrowing this approach to deliver drugs through the gut.
Sign up for Nature’s newsletter on robotics and AI
Solid tumours are another difficult place to deliver a drug. As these malignancies develop, a ravenous hunger for oxygen promotes an outside surface covered with blood vessels, while an oxygen-deprived core builds up within. Low oxygen levels force cells deep inside to switch to anaerobic metabolism and churn out lactic acid, creating acidic conditions. As the oxygen gradient builds, the tumour becomes increasingly difficult to penetrate. Nanoparticle drugs lack a force with which to muscle through a tumour’s fortifications, and typically less than 2% of them will make it inside8. Proponents of nanomachines think that they can do better.
Sylvain Martel, a nanoroboticist at Montreal Polytechnic in Canada, is trying to break into solid tumours using bacteria that naturally contain a chain of magnetic iron-oxide nanocrystals. In nature, these Magnetococcus species seek regions that have low oxygen. Martel has engineered such a bacterium to target active cancer cells deep inside tumours8. “We guide them with a magnetic field towards the tumour,” explains Martel, taking advantage of the magnetic crystals that the bacteria typically use like a compass for orientation. The precise locations of low-oxygen regions are uncertain even with imaging, but once these bacteria reach the right location, their autonomous capability kicks in and they motor towards low-oxygen regions. In a mouse, more than half the bacteria injected close to tumour grafts broke into this tumour region, each laden with dozens of drug-loaded liposomes. Martel cautions, however, that there is still some way to go before the technology is proven safe and effective for treating people with cancer.
In the Netherlands, chemist Daniela Wilson at Radboud University in Nijmegen and colleagues have developed enzyme-driven nanomotors powered by DNA that might similarly be able to autonomously home in on tumour cells9. The motors navigate towards areas that are richer in DNA, such as tumour cells that undergoing apoptosis. “We want to create systems that are able to sense gradients by different endogenous fuels in the body,” Wilson says, suggesting that the higher levels of lactic acid or glucose typically found in tumours could also be used for targeting. Once in place, the autonomous bots seem to be picked up by cells more easily than passive particles are — perhaps because the bots push against cells.

Fiction versus reality
Inspirational though Fantastic Voyage might have been for many working in the field of medical nanorobotics, there are some who think the film has become a burden. “People think of this as science fiction, which excites people, but on the other hand they don’t take it so seriously,” says Martel. Fischer is similarly jaded by movie-inspired hype. “People sometimes write very liberally as if nanobots for cancer treatment are almost here,” he says. “But this is not even in clinical trials right now.”
Nonetheless, advances in the past ten years have raised expectations of what is possible with current technology. “There’s nothing more fun than building a machine and watching it move. It’s a blast,” says Nelson. But having something wiggling under a microscope no longer has the same draw, without medical context. “You start thinking, ‘how could this benefit society?’” he says.
With this in mind, many researchers creating nanorobots for medical purposes are working more closely with clinicians than ever before. “You find a lot of young doctors who are really interested in what the new technologies can do,” Nelson says. Neurologist Philipp Gruber, who works with stroke patients at Aarau Cantonal Hospital in Switzerland, began a collaboration with Nelson two years ago after contacting ETH Zurich. The pair share an ambition to use steerable microbots to dissolve clots in people’s brains after ischaemic stroke — either mechanically, or by delivering a drug. “Brad knows everything about engineering,” says Gruber, “but we can advise about the problems we face in the clinic and the limitations of current treatment options.”
Sánchez tells a similar story: while he began talking to physicians around a decade ago, their interest has warmed considerably since his experiments in animals began three to four years ago. “We are still in the lab, but at least we are working with human cells and human organoids, which is a step forward,” says his collaborator Vilaseca.
As these seedlings of clinical collaborations take root, it is likely that oncology applications will be the earliest movers — particularly those that resemble current treatments, such as infusing microbots instead of BCG into cancerous bladders. But even these therapeutic uses are probably at least 7–10 years away. In the nearer term, there might be simpler tasks that nanobots can be used to accomplish, according to those who follow the field closely.
For example, Martin Pumera, a nanoroboticist at the University of Chemistry and Technology in Prague, is interested in improving dental care by landing nanobots beneath titanium tooth implants10. The tiny gap between the metal implants and gum tissue is an ideal niche for bacterial biofilms to form, triggering infection and inflammation. When this happens, the implant must often be removed, the area cleaned, and a new implant installed — an expensive and painful procedure. He is collaborating with dental surgeon Karel Klíma at Charles University in Prague.
Another problem the two are tackling is oral bacteria gaining access to tissue during surgery of the jaws and face. “A biofilm can establish very quickly, and that can mean removing titanium plates and screws after surgery, even before a fracture heals,” says Klíma. A titanium oxide robot could be administered to implants using a syringe, then activated chemically or with light to generate active oxygen species to kill the bacteria. Examples a few micrometres in length have so far been constructed, but much smaller bots — only a few hundred nanometres in length — are the ultimate aim.
Clearly, this is a long way from parachuting bots into hard-to-reach tumours deep inside a person. But the rising tide of in vivo experiments and the increasing involvement of clinicians suggests that microrobots might just be leaving port on their long journey towards the clinic.
doi: https://doi.org/10.1038/d41586-022-00859-0
This article is part of Nature Outlook: Robotics and artificial intelligence, an editorially independent supplement produced with the financial support of third parties. About this content.
References
- Ghosh, A. & Fischer, P. Nano Lett. 9, 2243–2245 (2009).PubMed Article Google Scholar
- Wu, Z. et al. Sci. Adv. 4, eaat4388 (2018).PubMed Article Google Scholar
- Gao, C. et al. ACS Appl. Mater. Interfaces 11, 23392–23400 (2019).PubMed Article Google Scholar
- Xu, H. et al. ACS Nano 12, 327–337 (2018).PubMed Article Google Scholar
- Yan, X. et al. Sci. Robot. 2, eaaq1155 (2017).PubMed Article Google Scholar
- Hortelao, A. C. et al. Sci. Robot. 6, eabd2823 (2021).PubMed Article Google Scholar
- Gao, W. et al. ACS Nano 9, 117–123 (2015).PubMed Article Google Scholar
- Felfoul, O. et al. Nature Nanotechnol. 11, 941–947 (2016).PubMed Article Google Scholar
- Ye, Y. et al. Nano Lett. 21, 8086–8094 (2021).PubMed Article Google Scholar
- Villa, K. et al. Cell Rep. Phys. Sci. 1, 100181 (2020).Article Google Scholar




